Katie Bush of Avita Medical, PHD, analyzes how the Recel skin regeneration system is helping plastic surgeons in hospitals and clinicians to reduce the loading of the donor site, accelerate healing and improve the results in the care of acute wounds and reconstructive procedures.
Cell -based regenerative technologies are expanding the options available for plastic surgeons working in hospital and clinical environments. The best Reell Medical Avita system for its use in acute burning care has treated more than 30,000 patients from the approval of the FDA and now applies to a broader range of reconstructive procedures, including scars reviews, trauma cases and after. By creating a suspension of spraying of the skin of a patient from a small sample of donors, Recel allows the treatment of larger surface areas while minimizing the morbidity and recovery time of the donor site.
The recent authorizations of the FDA, including CPT codes of category I and the new clinical indications, are positioning Rell for broader use beyond burning centers. To discuss these development, Practice of Plastic Surgery He spoke with Katie Bush, PHD, senior vice president of scientific and medical matters in Avita Medical. She shares how Rell is evolving and what her expanding role for plastic and reconstructive surgery means.
Practice of plastic surgery: Rell has made significant advances in burning care: Does it show that its application evolves in elective and reconstructive plastic surgery environments?
Katie Bush, pHD: With more than 30,000 patients treated as a FDA approach, Rell’s success in burning care has built a solid base for use. We see enormous potential in procedures such as scars reviews, trauma reconstructions and postcological split repairs. The ability to generate a regenerative spray of skin cells using a small skin sample allows minimizing the morbidity of the donor site while improving the results in terms of speed to the recovery of the patient and the reduction of the length or the stay in the hospital.
PSP: For plastic surgeons, what are the most convincing clinical advantages offered by Rell compared to the traditional autogeefting techniques?
Bush: The most convincing advantages of Rell are the reduced load of the donor site (donor tissue size, pain in the donor site and better aesthetic results), accelerated healing and reduction in the length or stay in the hospital. Rell allows the treatment of larger surface areas with significantly smaller donor sites compared to traditional leather graft techniques, which translates into less pain and faster recovery for patients. Surgeons also appreciate their flexibility. Rell can be used only in partial burns or in combination with mesh grafts for full task injuries.
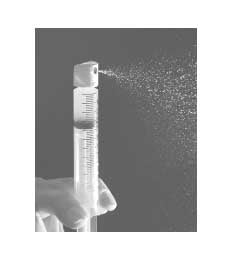

PSP: Can you guide us through the mechanism of action behind the skin with Aerosol de Rell and how improves wound healing and aesthetic results?
Bush: Rell’s spray skin offers a suspension of keratinocytes, melanocytes and fibroblasts, including skin keratinocytes. These cells are key promoters of the healing and restoration of the pigment. When quickly repopularly with the wound with types of thesis cells, Recel admits a faster closure at the cellular level through the entire surface of the wound. It is known that faster healing results in less scars.
PSP: What does the current body of clinical evidence say about the quality of the scar and patient satisfaction with conventional skin graft?
Bush: In a random controlled trial, it was reported that the results of the scar and the pain informed at the donor site are more favorable for the Recel treatment group compared to the standard leather graft group. Long -term evaluations showed that the subjects expressed greater satisfaction with the visual appearance of the Donor Reell site compared to the control site.
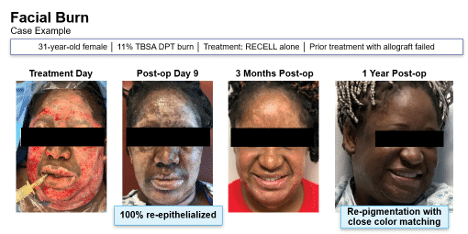

PSP: Are there populations of specific patients or types of cases where Rell has demonstrated a particular value?
Bush: Yes, Rell has shown value in patient populations where the availability of the donor site is limited, preservation is a clinical priority and a minimal invasiveness is essential. This includes patients with fragile or aging skin, and those with comorbidities that compromise wound healing. Rell is also special advantage in cases that require great surface area coverage of a small harvest of donors, such as traumatic lesions or complex reconstructive procedures.
In general, Rell’s ability to significantly reduce the donor site load, while the effective promotion reepitelization makes it a highly versatile tool that should be considered for all patient populations.
PSP: How steep is the learning curve to integrate Recel in the practice of plastic surgery and what kind of support does Avita offer for adoption?
Bush: Avita Medical offers comprehensive training through workshops in person, education between peers and practical support, which helps surgeons to be competent quickly. We also provide clinical orientation and ONDOG reimbursement to guarantee a soft transition and successful integration in the workflows of the practice.
PSP: As the regulations and reimbursement models change, how is the positioning of avita that is suspicious to continue being viable and attacked for use outside the large hospital systems?
Bush: Avita is taking deliberate measures to support the use of Rell in a variety of care settings. In January 2025, the new CPT categories of category I specific to the Self -Cell Cell Suspension Procedures (SCSA) use the tok effect of the Recel System, providing a defined coding pathway and enabling reimbursement for both facilities and doctors. Medicare recognizes and reimburses Recel, and we are actively working with commercial payers to expand coverage as outpatient adoption grows.
Combined with the ease of use of the system and the minimum infrastructure requirements, these efforts are recording to continue being a viable and accessible treatment option beyond large hospital systems.


PSP: Looking towards the future, what innovations or clinical indications are exploring Avita that could further extend Rell’s relevance in the field of plastic surgery?
Bush: Recently, Avita Medical introduced the next -generation Reell Go device that has an updated interface designed to admit a broader adoption in a range of hospital environments. Avita Medical has also introduced the Rell Go Mini Disposable cartridge designed to specifically treat narrower wounds up to 2.5% of the total surface area of the body, compared to the standard recell that can be discarded, which deals with an area or up to 10%. Rell Go Mini addresses a critical need in the plastic surgery market today to obtain Rell’s benefits by treating a large volume of smaller wounds.
In addition, Avita Medical is expanding its portfolio with two recently approved complementary products to claim. The first product is Permeaderm, a temporary biosynthetic dressing used to imitate the epidermal layer of the skin until healing can be achieved. We are currently recruiting for Pereseaderm I, a controlled randomized prospective study that evaluates the use of prepeaderm as a temporary dressing compared to the cadaveric fabric. Cohealyx, our addition of products, are the wounds of dermal collagen-t. In a preclinical study, Cohelyx supported cell migration and revascularization, creating a bed of the wound ready for skin graft in 7 days. We are seeing similar results in clinical use and we have a clinical trial of ONS, Coheallyx I, formally evaluating the preparation of the bed of wounds, self -government time, autographs taken, healing and advertising events related to safety. PSP
]


